Key to understanding ovarian cancer metastasis could lie in cancer cells acting in groups
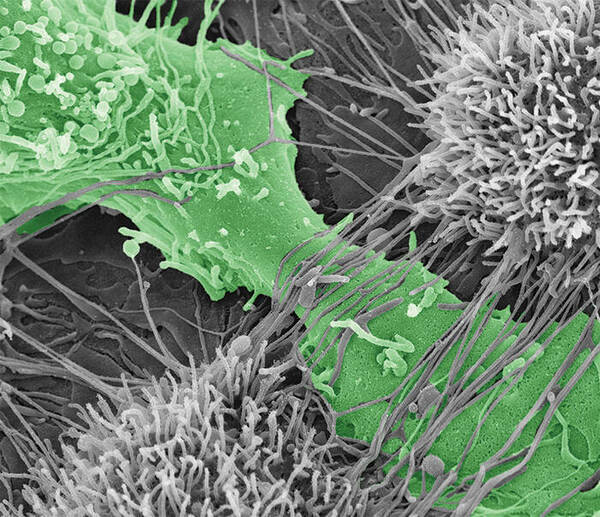
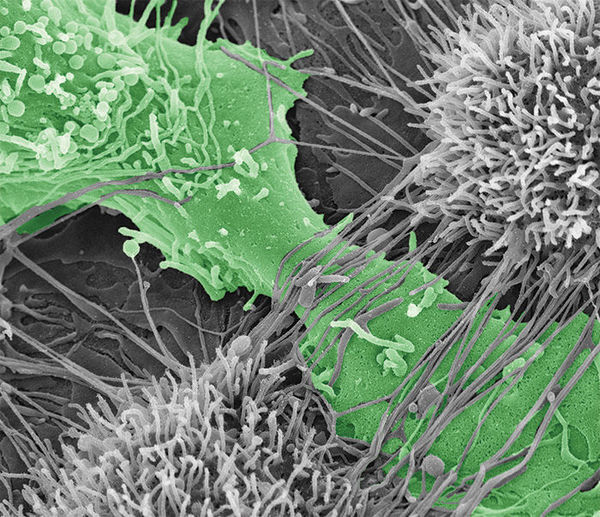 Ovarian cancer cell tearing apart the mesothelial layer of the peritoneum.
Ovarian cancer cell tearing apart the mesothelial layer of the peritoneum.
When it comes to ovarian cancer, 60 percent of patients are diagnosed in stage III, meaning the cancer has already metastasized, or spread, throughout the pelvis. Additionally, between 70 and 90 percent of those patients will be diagnosed with recurrence and although recurrent ovarian cancer is treatable, it is rarely curable. These unfortunate results are partially due to the disease’s ability to spread cancer cells and therefore efficiently penetrate other organs. To better understand how metastatic ovarian cancer spreads, Notre Dame researchers at the Harper Cancer Research Institute (HCRI) are evaluating the impact of ovarian cancer cell molecular composition and how these cells work together to invade surrounding tissue.
Yuliya Klymenko, a postdoctoral scholar in chemistry & biochemistry, recent Fulbright awardee, and affiliated member of the HCRI, led two studies evaluating the role multicellular aggregates (MCAs) – groups of cancer cells that are clustered together – play in ovarian cancer metastasis. In discussing the research, Klymenko said, “Ovarian cancer is unique compared to other cancers in that when cells shed off of a tumor, they don’t die. Instead, the cells survive in the peritoneal cavity – a space filled with fluid that includes the abdominal and pelvic areas. In order to spread disease, the cells rely on cadherins, a type of adhesion molecule, which make the cells bind together into MCAs. Cadherins not only give integrity to the MCAs, but also help the cancer cells stick to and penetrate tissue.”
In a study published in Neoplasia, funded by the National Institutes for Health (NIH) and the affiliated National Cancer Institute, the Notre Dame researchers looked at how ovarian cancer cells group differently based on the type of cadherin molecule the cells expressed. The research assessed three different types of cadherin expression patterns in cancer cells: N-cadherin, E-cadherin, and a hybrid with both N- and E-cadherin. The study showed that cells with the same cadherin make-up were drawn to each other and that cadherin composition can actually regulate the MCAs’ compaction, cohesiveness, and ultimate dissemination. More importantly, these results suggest that the type of cadherin expressed by MCAs could change how the cells respond to therapeutics.
“Although MCAs are recognized as a key component to spreading ovarian cancer, most intervention or treatment research is still conducted on single cancer cells,” said M. Sharon Stack, the Anne F. Dunne and Elizabeth Riley Director of the HCRI, the Kleiderer-Pezold Professor of Chemistry & Biochemistry, and principal investigator on the research. “However, our results show that new therapeutic interventions may actually lie in targeting different cadherin compositions because not only do cadherin molecules help determine MCA behavior, but single-cell tested treatments may only impact the outer cells of MCAs, leaving the remaining, inner group of cells to spread to other tissue.”
To predict how different cadherin compositions can regulate metastasis, it is first important to determine how MCAs expressing N-, E-, and hybrid cadherin molecules differ in their contribution to spreading ovarian cancer to other tissue. In a study published in Oncogene, Klymenko, Stack, and their team evaluated the effectiveness of MCAs’ ability to destroy the mesothelial layer, or top layer, of the peritoneum – the membrane that lines the abdomen, covers all abdominal organs, and is the initial point of contact for cancer cells after shedding from an ovarian cancer tumor. Additionally, they wanted to compare how different cadherin compositions varied the speed of which cancer penetrated collagen below the mesothelial layer.
The NIH-funded study used live cell imaging microscopy to compare how individual N- or E-cadherin-expressing cells invaded into collagen relative to N- or E-cadherin-expressing MCAs. The results showed that N-cadherin MCAs were significantly more aggressive in their mesothelial clearing process and penetration of the collagen layer. The study also tested how an N-cadherin-blocking antibody performed against N-cadherin cells, and showed promising results.
“Knowing specific details of varying cellular processes is essential for creating targeted therapies, especially since recurring ovarian cancer can be more resistant to medication,” said Klymenko. “Through this research, we have provided rationale that supports future pre-clinical studies using intra-peritoneal delivery of N-cadherin blocking molecules as a therapeutic for ovarian cancer metastasis.”
In addition to Klymenko and Stack, the research published in the journal Neoplasia was conducted with Jeffrey Johnson, senior scientist at HCRI; Notre Dame undergraduates Brandi Bos, ‘17, Rachel Lombard, ‘17; Leigh Campbell, ’19; and Elizabeth Loughran, graduate student of chemistry and biochemistry and affiliated member of the HCRI. The research published in Oncogene was conducted with Oleg Kim, assistant research scientist of applied mathematics at University of California, and affiliated member of the HCRI; Lombard; Loughran; Jing Yang, research scientist at the HCRI; and Mark Alber, professor emeritus of applied and computational mathematics and statistics at Notre Dame, as well as professor of applied mathematics at the University of California.
In addition to being funded through the NIH, the studies were also funded through the Leo and Anne Albert Charitable Trust, the Research Like a Champion grant, the Walther Cancer Foundation Seeding Research in Cancer grant, and the National Science Foundation.
HCRI researchers are dedicated to conducting innovative and integrative research that confronts the complex challenges of cancer. From common malignancies to rare and recalcitrant cancers, researchers at the University of Notre Dame and Indiana University School of Medicine – South Bend are united in multi-disciplinary teams with a common goal: to increase the survival of all patients diagnosed with cancer.
To learn more about how the University of Notre Dame fights cancer, please visit harpercancer.nd.edu.
Originally published by at research.nd.edu on July 05, 2017.